Quantum Numbers for Atoms - Chemistry LibreTexts
By A Mystery Man Writer
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is …
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is described by a wave function that complies with the Schrödinger equation. Each electron in an atom has a unique set of quantum numbers; according to the Pauli Exclusion Principle, no two electrons can share the same combination of four quantum numbers.

Quantum Numbers for Atoms - Deepstash

Difference Between Px Py and Pz Orbitals

Science Activity Sheet: Quarter 2 - MELC 1 Week 1, PDF, Atomic Orbital

Impressions: Robinson's Brutus Awards For 2015, Part, 42% OFF

What Are Quantum Numbers? What Are Rules For Electron Configuration?
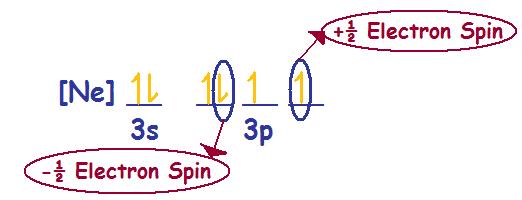
Electron Spin - Chemistry LibreTexts

Transition metal Definition, Properties, Elements, & Facts

Science Activity Sheet: Quarter 2 - MELC 1 Week 1, PDF, Atomic Orbital

Impressions: Robinson's Brutus Awards For 2015, Part, 42% OFF

Quantum Numbers for Atoms - Chemistry LibreTexts

Quantum Numbers and Electron Configurations

Las shs gen.chem-melc_1_q2_week-1

Las shs gen.chem-melc_1_q2_week-1
:max_bytes(150000):strip_icc()/Getty_English_as_a_lingua_franca-179708542-56afa5465f9b58b7d01b7464.jpg)


:max_bytes(150000):strip_icc()/VWH_Illustration_Lice-vs-Dandruff_Illustrator_Jessica-Olah_Final-c2895a2e233f47edb444f88b53d864e9.jpg)